Practical biocatalytic desymmetrization of meso-N-heterocyclic dicarboxamides and their application in the construction of aza-sugar containing nucleoside analogs†
Abstract
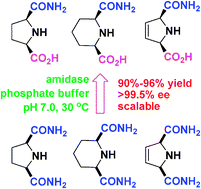
Amidase-catalyzed desymmetrization of meso-N-heterocyclic dicarboxamides under very mild conditions provided a highly efficient and practical method for the preparation of enantiomerically pure

 Please wait while we load your content...
Please wait while we load your content...