A novel C-5′ substituted cinchona alkaloid-derived catalyst promotes additions of alkyl thiols to nitroolefins with excellent enantioselectivity†‡
Abstract
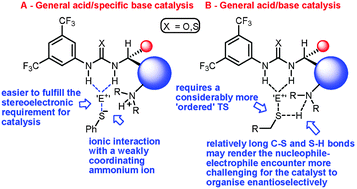
A new bifunctional C-5′ substituted
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...