Highly enantioselective Mukaiyama aldol reaction in aqueous conditions using a chiral iron(ii) bipyridine catalyst†
Abstract
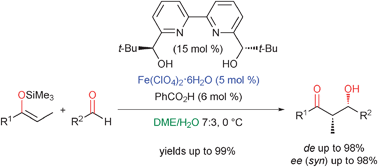
A highly enantioselective method for the catalytic Mukaiyama aldol reaction of
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...