Supramolecular assembling systems formed by heme–heme pocket interactions in hemoproteins
Abstract
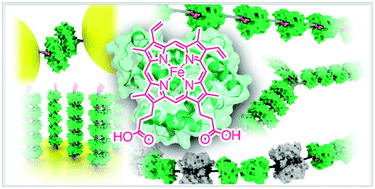
A native protein in a biological system spontaneously produces large and elegant assemblies via self-assembly or assembly with various biomolecules which provide non-covalent interactions. In this context, the protein plays a key role in construction of a unique supramolecular structure operating as a functional system. Our group has recently highlighted the structure and function of hemoproteins reconstituted with artificially created heme analogs. The heme molecule is a replaceable cofactor of several hemoproteins. Here, we focus on the successive supramolecular protein assemblies driven by heme–heme pocket interactions to afford various examples of protein fibers, networks and three-dimensional clusters in which an artificial heme moiety is introduced onto the surface of a hemoprotein via covalent linkage and the native heme cofactor is removed from the heme pocket. This strategy is found to be useful for constructing hybrid materials with an electrode or with nanoparticles. The new systems described herein are expected to lead to the generation of various biomaterials with functions and characteristic physicochemical properties similar to those of hemoproteins.

 Please wait while we load your content...
Please wait while we load your content...