Tailor-made synthesis of various backbone-substituted imidazolinium salts by triflic anhydride mediated intramolecular cyclisation†
Abstract
We have found a Tf2O-mediated intramolecular cyclization reaction and have revealed an intriguing stereoselectivity and a regioselectivity during the preparation of intermediate
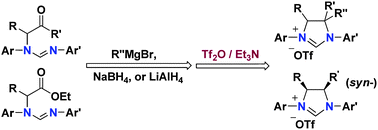

 Please wait while we load your content...
Please wait while we load your content...