A quantitative study of intrinsic non-covalent interactions within complexes of α-cyclodextrin and benzoate derivatives†
Abstract
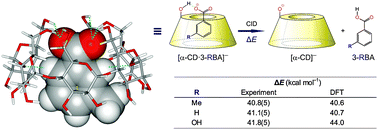
A novel deconvolution method for energy-resolved reaction cross sections is applied to determine intrinsic gas-phase dissociation energies for non-covalent α-cyclodextrin host–guest complexes. M06-2X//M06-L/6-31+G(d,p) calculations reproduce the experimental results and enable us to quantify the contribution of intermolecular hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...