Alkene oxidation by Ti-containing polyoxometalates. Unambiguous characterization of the role of the protonation state†
Abstract
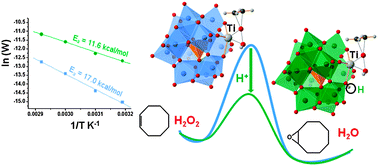
Kinetic and DFT studies revealed that protonation of Ti-containing polyoxometalates (Ti-POM) lowers significantly the energy barrier for the heterolytic oxygen transfer from the Ti hydroperoxo intermediate to the

 Please wait while we load your content...
Please wait while we load your content...