gem-Dialkylthio vinylallenes: alkylthio-regulated reactivity and application in the divergent synthesis of pyrroles and thiophenes†
Abstract
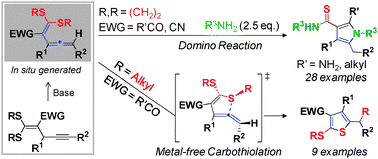
gem-Dialkylthio vinylallenes were obtained for the first time and applied to the divergent synthesis of fully-substituted

 Please wait while we load your content...
Please wait while we load your content...