Chiral proton catalysis of secondary nitroalkane additions to azomethine: synthesis of a potent GlyT1 inhibitor†‡
Abstract
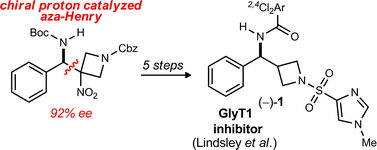
The first enantioselective synthesis of a potent GlyT1 inhibitor is described. A 3-nitroazetidine donor is used in an enantioselective aza-Henry reaction catalyzed by a bis(amidine)-triflic acid salt organocatalyst, delivering the key intermediate with 92% ee. This adduct is reductively denitrated and converted to the target through a short sequence, thereby allowing assignment of the absolute configuration of the more potent enantiomer.
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...