Sorption and separation of CO2via nanoscale AlO(OH) hollow spheres†
Abstract
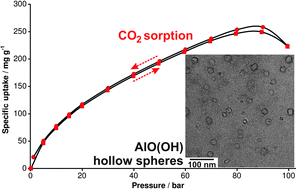
The CO2 uptake on nanoscale AlO(OH) hollow spheres (260 mg g−1) as a new material is comparable to that on many metal–organic frameworks although their specific surface area is much lower (530 m2 g¬1versus 1500–6000 m2g¬1). Suited temperature–pressure cycles allow for reversible storage and separation of CO2 while the CO2 uptake is 4.3-times higher as compared to N2.

 Please wait while we load your content...
Please wait while we load your content...