Development and validation of a rapid HPLC method for the determination of ascorbic acid, phenylephrine, paracetamol and caffeine using a monolithic column†
Abstract
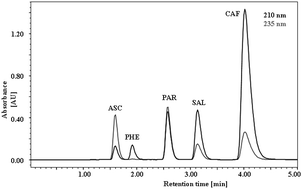
This article reports a fast and simple liquid chromatographic method for determination of ascorbic acid, phenylephrine, paracetamol and caffeine. Salicylic acid was used as internal standard. The analytes were successfully separated in less than 5 min by isocratic elution using monolithic column, Onyx Monolithic C18 (100 × 4.6 mm), with mobile phase composed of acetonitrile and phosphate buffer (pH 6.50) (10 : 90, v/v) at a flow rate of 1.0 mL min−1 and 25 °C, sample volume was 10 μL. Detection was observed at two wavelengths 210 nm (phenylephrine, paracetamol and salicylic acid) and 235 nm (ascorbic acid and caffeine). The optimized method was applied for the determination of the analytes in pharmaceutical formulation Coldrex tablets (Smithkline Beecham Consumer Healthcare, United Kingdom) commonly used in virosis treatment.
- This article is part of the themed collection: Pharmaceutical Analysis

 Please wait while we load your content...
Please wait while we load your content...