Inorganic–organic Ag-rhodamine 6G hybrid nanorods: “Turn on” fluorescent sensors for highly selective detection of Pb2+ ions in aqueous solution
Abstract
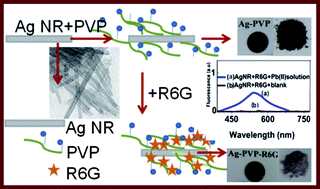
Lead metal ions are of great concern and the monitoring of their concentration in the environment has become extremely important. In the present study, a new inorganic–organic hybrid assay of Ag

 Please wait while we load your content...
Please wait while we load your content...