Colorimetric detection of copper ions in sub-micromolar concentrations using a triarylamine-linked resin bead†
Abstract
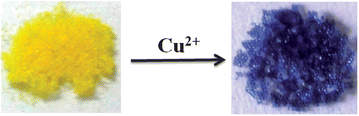
The triarylamine derivative ETPA reacts with Cu2+ to give deeply colored, stable radical cations in acetonitrile solution. ETPA was immobilized on to a tentagel resin bead which was then used for the fabrication of a simple device capable of the colorimetric detection of submicromolar concentrations of Cu2+ ions in water. The naked eye detection limit reported here for Cu2+ is one of the lowest ever reported for small molecule sensors.

 Please wait while we load your content...
Please wait while we load your content...