Facile growth factor immobilization platform based on engineered phage matrices†
Abstract
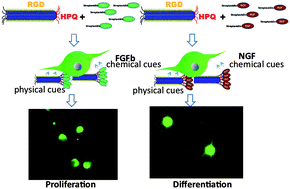
Immobilized growth factors on tissue matrices play a critical role in controlling cell growth processes and cell morphology. We report a strategy for immobilizing growth factors on genetically engineered phage matrices for tissue regeneration. We modified M13 phages to express

 Please wait while we load your content...
Please wait while we load your content...