Reversible tuning of the hydrophobic–hydrophilic transition of hydrophobic ionic liquids by means of an electric field†
Abstract
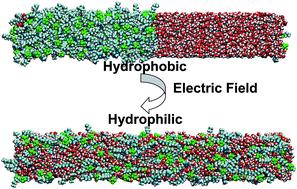
In this work, we demonstrate for the first time that hydrophobic ILs can be strikingly tuned to be hydrophilic under a strong external electric field, with the use of nonequilibrium molecular dynamics (MD) simulations and

 Please wait while we load your content...
Please wait while we load your content...