Nickel-catalyzed amination of aryl carbamates and sequential site-selective cross-couplings†
Abstract
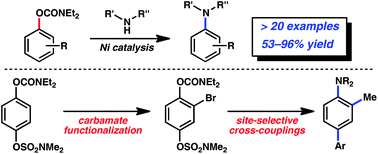
We report the amination of aryl carbamates using nickel-catalysis. The methodology is broad in scope with respect to both coupling partners and delivers aminated products in synthetically useful yields. Computational studies provide the full catalytic cycle of this transformation, and suggest that reductive elimination is the rate-determining step. Given that carbamates are easy to prepare, robust, inert to Pd-catalysis, and useful for

 Please wait while we load your content...
Please wait while we load your content...