Catalytic capsids: the art of confinement†
Abstract
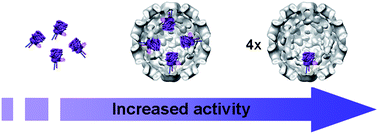
In the cell, enzymes are almost always spatially confined in crowded and tightly controlled cellular compartments. The entrapment of enzymes in artificial nanoreactors as biomimetic systems can be expected to contribute to the understanding of the activity and the interactions of enzymes in confined spaces. The capsid of the Cowpea Chlorotic Mottle virus (CCMV) represents such an artificial nanoreactor that can be used to encapsulate multiple

 Please wait while we load your content...
Please wait while we load your content...