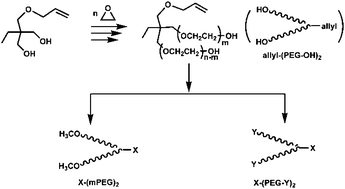
Allyl-(PEG-OH)2, a dual-branched poly(ethylene glycol) (PEG) with a latent group amenable for modification at the junction, was successfully synthesized using trimethylolpropane allyl ether (TMPAE), a commercially available compound, as the initiator for anionic polymerization. To demonstrate the versatility of this approach, derivatives of the branched PEG were formed using simple modification. The chain ends of PEG were modified to inert methoxy groups and active functional groups. Using an orthogonal reaction procedure, the allyl junction was modified to carboxyl and amino group. The synthesis route was short, quantitative, and easily controlled. No cumbersome purification was needed. The branched PEG and its derivatives were characterized by SEC, 1H and 13C NMR, and MALDI-TOF mass spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...