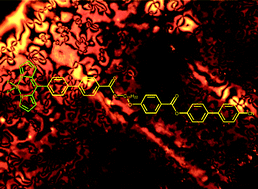
A series of mesogenic molecules based on the 4,4′-difluoro-3a,4a-diaza-s-indacene (BODIPY) fluorophore have been synthesised and characterised. Each compound consists of one mesogenic unit, based on a cyanobiphenyl core, and one fluorophore, of varying alkyl substitution. The compounds were prepared by microwave-assisted palladium-catalyzed couplings (Suzuki and Sonogashira) due to slow reaction rates under conventional heating conditions. The effect of increasing the molecular length was also investigated by incorporating an ethynyl unit between the mesogen and the fluorophore. The molecules self-assemble into monotropic nematic phases which were identified by optical polarising microscopy (OPM) and differential scanning calorimetry (DSC). The compounds were found to display varying degrees of fluorescence quantum yield dependant on the number of alkyl substituents attached to the fluorophore. A relationship was observed between the nematic phase stability and fluorescence intensity of the compounds with increasing alkyl substitution causing an increase in fluorescence intensity due to restriction of the 8-phenyl ring rotation while simultaneously disrupting nematic phase formation, causing a reduction in nematic range. Temperature-dependant fluorescence measurements were also acquired along with fluorescence measurements of four of the synthesised BODIPYs dissolved in a commercial nematic liquid crystal (BL024) and incorporated into a twisted nematic cell.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...