Synthetic approaches for the conjugation of porphyrins and related macrocycles to peptides and proteins†
Abstract
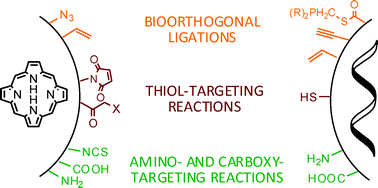
The association of photosensitisers to
- This article is part of the themed collection: Drug delivery technologies and immunological aspects of photodynamic therapy

 Please wait while we load your content...
Please wait while we load your content...