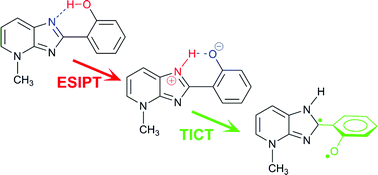
The ground- and excited-state behaviour of the isomeric species 2-(2′-methoxyphenyl)imidazo[4,5-b]pyridine (1-OMe) and 2-(2′-hydroxyphenyl)-4-methylimidazo[4,5-b]pyridine (1-NMe) in neutral and acid media has been studied by UV-vis absorption spectroscopy, steady-state and time-resolved fluorescence spectroscopy. The new dye 1-NMe is non-fluorescent in neutral media except in trifluoroethanol, where it shows a very weak fluorescence. 1-NMe also exhibits highly solvent-dependent fluorescence intensity in acidic media. We propose that the neutral species experiences a fast excited-state intramolecular proton transfer (ESIPT), relaxing afterwards by intramolecular twisting associated with internal charge transfer (TICT) and subsequent very fast internal conversion of the proton-transferred TICT structure. The behaviour of 1-NMe in acidic media is explained by the existence of a ground-state tautomeric equilibrium between species with intramolecular hydrogen bonds N–H⋯OH and N⋯HO. The first type of tautomers dissociates at the hydroxyl group in water and ethanol, but fluoresces in acetonitrile and trifluoroethanol due to the inability of these solvents to accept the proton. The second type of tautomers is non-emissive due to fast radiationless deactivation through an ESIPT-TICT process. The fluorescence of 1-OMe was investigated in neutral and acidic media, demonstrating the photobasic character of the pyridine nitrogen. A ground-state equilibrium between pyridinium and imidazolium cations was found for this species, showing overlapping absorption and fluorescence spectra. We devised a method to resolve the spectra by applying principal component global analysis to a series of excitation spectra taken at different emission wavelengths, which allowed estimation of the equilibrium constant between the cations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...