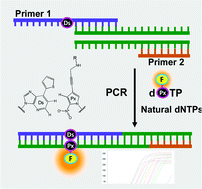
We developed intramolecular dual fluorophore-quencher base analogues for site-specific incorporation into DNA by an unnatural base pair replication system. An unnatural base pair between 7-(2-thienyl)-imidazo[4,5-b]pyridine (Ds) and 2-nitro-4-propynylpyrrole (Px) exhibits high fidelity in PCR amplification, and the 2-nitropyrrole moiety of Px acts as a quencher. Deoxyribonucleoside triphosphates of Px linked with a fluorophore (Cy3, Cy5 or FAM) were chemically synthesized, and the fluorescent properties and the enzymatic incorporation of the fluorophore-linked dPxTPs into DNA were examined in PCR amplification. The fluorophore-linked dPxTPs were site-specifically incorporated by PCR into DNA, opposite Ds in templates, with high selectivity. Furthermore, we found that the fluorescence of the triphosphates was partially quenched, but increased upon their incorporation into DNA. These dual fluorophore-quencher base analogues would be useful for site-specific DNA labeling and for monitoring the amplification products of target nucleic acid molecules with a specific sequence. We have demonstrated the utility of the fluorophore-linked Px substrates and the Ds-Px pairing in real-time quantitative PCR for target DNA molecule detection.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...