New synthetic route to substituted dihydroazulene photoswitches†
Abstract
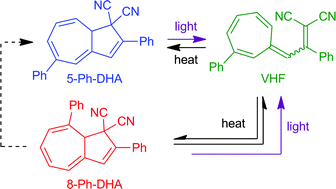
A new procedure for functionalization of the dihydroazulene photoswitch on its seven-membered ring was developed, which has allowed isolation of the first dihydroazulene with a phenyl substituent at position 5 from a mixture of regioisomers. Light-induced ring-opening to the corresponding vinylheptafulvene and the thermal back-reaction was studied in detail.
 Please wait while we load your content...
Please wait while we load your content...