Modular synthesis of 1-α- and 1-β-(indol-2-yl)-2′-deoxyribose C-nucleosides†
Abstract
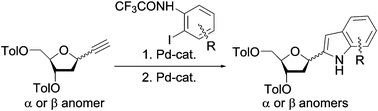
A simple two-step method for the selective preparation of anomerically pure 1α- and 1β-(indol-2-yl)deoxyribose derivatives was developed. The synthesis was based on the Sonogashira reaction of 1α- and 1β-ethynyldeoxyribose and 2-haloanilines followed by a Pd-complex catalyzed

 Please wait while we load your content...
Please wait while we load your content...