Bent bonds, the antiperiplanar hypothesis and the theory of resonance. A simple model to understand reactivity in organic chemistry
Abstract
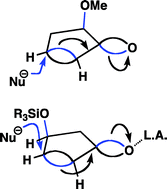
By taking into consideration bent bonds (τ-bonds, tau-bonds), the antiperiplanar hypothesis, the classic theory of resonance, and the preference for staggered bonds over eclipsed bonds in tetrahedral systems, a simple qualitative model is presented to rationalize the conformation and reactivity for a wide range of compounds containing double bonds and/or

 Please wait while we load your content...
Please wait while we load your content...