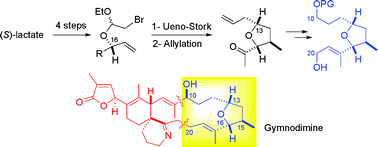
Abstract
A straightforward access to the C10–C20 skeleton of gymnodimine, incorporating a tetrahydrofuran fragment, is described. The elaboration of the THF moiety is based on a stereocontrolled Ueno–Stork cyclization. A Lewis-acid mediated allylation of the resulting
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...