Intermolecular radical addition to N-acylhydrazones as a stereocontrol strategy for alkaloid synthesis: formal synthesis of quinine†‡
Abstract
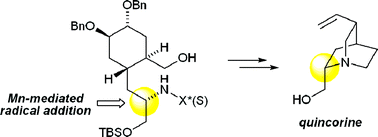
Stereocontrolled Mn-mediated radical addition of alkyl iodides to chiral N-acylhydrazones enables strategic C–C bond disconnection of chiral

 Please wait while we load your content...
Please wait while we load your content...