Enantioselective radical cyclisation reactions of 4-substituted quinolones mediated by a chiral template†
Abstract
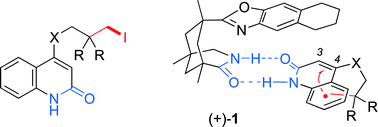
Six 4-substituted
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...