Mechanistic investigations on the efficient catalytic decomposition of peroxynitrite by ebselen analogues†
Abstract
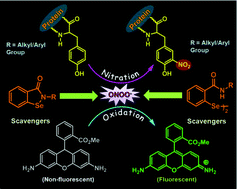
In this study, ebselen and its analogues are shown to be
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST
 Please wait while we load your content...
Please wait while we load your content...