Design and synthesis of amidine-type peptide bond isosteres: application of nitrile oxide derivatives as active ester equivalents in peptide and peptidomimetics synthesis†
Abstract
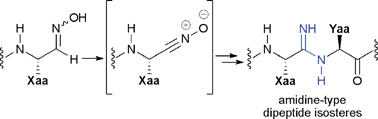
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group with an
O) group with an ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH) group. The positively-charged property of the isosteric part resembles a reduced
NH) group. The positively-charged property of the isosteric part resembles a reduced

 Please wait while we load your content...
Please wait while we load your content...