Aggregation behaviour of peptide–polymer conjugates containing linear peptide backbones and multiple polymer side chains prepared by nitroxide-mediated radical polymerization†‡
Abstract
A series of
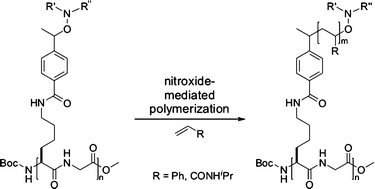
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...