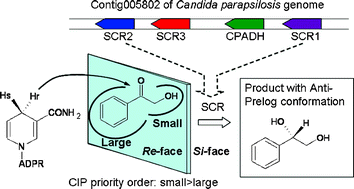
The application of biocatalysis to the synthesis of chiral molecules is one of the greenest technologies for the replacement of chemical routes due to its environmentally benign reaction conditions and unparalleled chemo-, regio- and stereoselectivities. We have been interested in searching for carbonyl reductase enzymes and assessing their substrate specificity and stereoselectivity. We now report a gene cluster identified in Candida parapsilosis that consists of four open reading frames including three putative stereospecific carbonyl reductases (scr1, scr2, and scr3) and an alcohol dehydrogenase (cpadh). These newly identified three stereospecific carbonyl reductases (SCRs) showed high catalytic activities for producing (S)-1-phenyl-1,2-ethanediol from 2-hydroxyacetophenone with NADPH as the coenzyme. Together with CPADH, all four enzymes from this cluster are carbonyl reductases with novel anti-Prelog stereoselectivity. SCR1 and SCR3 exhibited distinct specificities to acetophenone derivatives and chloro-substituted 2-hydroxyacetophenones, and especially very high activities towards ethyl 4-chloro-3-oxobutyrate, a β-ketoester with important pharmaceutical potential. Our study also showed that genomic mining is a powerful tool for the discovery of new enzymes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...