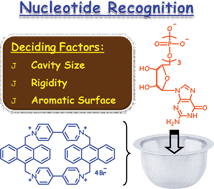
We synthesized a few novel cyclophanes CP-1 to CP-4 containing anthracene units linked together through different bridging and spacer groups and have investigated their interactions with various nucleosides and nucleotides. Of these systems, CP-1 and CP-3 showed selectivity for 5′-GTP and 5′-ATP as compared to other nucleotides and nucleosides, whereas negligible selectivity was observed with CP-2 and CP-4. Interestingly, CP-1, CP-2 and CP-3 exhibited significant binding interactions with the fluorescent indicator, 8-hydroxy-1,3,6-pyrene trisulfonate (HPTS), resulting in the formation of non-fluorescent complexes. Titration of these complexes with nucleosides and nucleotides resulted in the displacement of HPTS, leading to the revival of its fluorescence intensity. It was observed that 5′-GTP induced the maximum displacement of HPTS from the complex [CP-1·HPTS] with an overall fluorescence enhancement of ca. 150-fold, while 5′-ATP induced ca. 45-fold. Although the displacement of HPTS from the complexes [CP-2·HPTS] and [CP-3·HPTS] was found to be similar to that of [CP-1·HPTS], these complexes showed lesser selectivity and sensitivity. In contrast, negligible displacement of HPTS was observed from the complex [CP-4·HPTS] under similar conditions. These results indicate that CP-1, having a well-defined cavity and good electron acceptor (viologen), is capable of forming selective and stable complexes. Though CP-2 and CP-3 retain the good electron acceptor (viologen), their reduced aromatic surface and larger cavity, respectively, resulted in lesser sensitivity. In contrast, CP-4 having a large cavity and a poor acceptor (1,2-bis(pyridin-4-yl)ethene) showed negligible selectivity, thereby indicating the importance of cavity size, bridging unit and aromatic surface on biomolecular recognition properties of cyclophanes.

 Please wait while we load your content...
Please wait while we load your content...