Transition metal mediated construction of pyrrole ring on 2,3-dihydroquinolin-4(1H)-one: synthesis and pharmacological evaluation of novel tricyclic heteroarenes†
Abstract
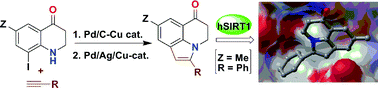
A facile two-step method for the construction of fused pyrrole ring leading to 5-substituted 2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinolin-1-ones via C–C followed by intramolecular C–N bond forming reaction is described. In vitro pharmacological evaluation and molecular modelling studies of some of the compounds synthesized are presented.

 Please wait while we load your content...
Please wait while we load your content...