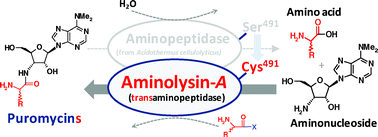
A new S9 family aminopeptidase derived from the actinobacterial thermophile Acidothermus cellulolyticus was cloned and engineered into a transaminopeptidase by site-directed mutagenesis of catalytic Ser491 into Cys. The engineered biocatalyst, designated aminolysin-A, can catalyze the formation of peptide bonds to give linear homo-oligopeptides, hetero-dipeptides, and cyclic dipeptides using cost-effective substrates in a one-pot reaction. Aminolysin-A can recognize several C-terminal-modified amino acids, including the L- and D-forms, as acyl donors as well as free amines, including amino acids and puromycin aminonucleoside, as acyl acceptors. The absence of amino acid esters prevents the formation of peptides; therefore, the reaction mechanism involves aminolysis and not a reverse reaction of hydrolysis. The aminolysin system will be a beneficial tool for the preparation of structurally diverse peptide mimetics by a simple approach.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...