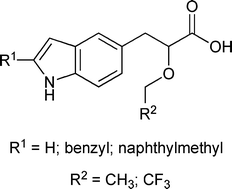
Peroxisome proliferator activated receptors (PPARs) have been shown to have critical roles in fatty acid oxidation, triglyceride synthesis, and lipid metabolism - making them an important target in drug discovery. Here we describe the in silico design, synthesis and in vitro characterisation of a novel series of 2,5-disubstituted indoles as PPARα/γ dual agonists. PPAR activation assays are performed with known agonists diazabenzene (WY14.643), aminopyridine (BRL49653) and bisaryl (L165.041), as positive controls. All the indole compounds synthesized are found to be active PPARα and PPARγ agonists, with particular efficacy from those with 2-naphthylmethyl substitution. This is a useful demonstration of a new de novo design methodology implemented by the PROTOBUILD program and its ability to rapidly produce novel modulators for a well characterized drug target.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...