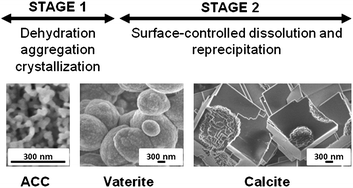
The kinetics and mechanisms of nanoparticulate amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite, were studied at a range of environmentally relevant temperatures (7.5–25 °C) using synchrotron-based in situ time-resolved Energy Dispersive X-ray Diffraction (ED-XRD) in conjunction with high-resolution electron microscopy, ex situX-ray diffraction and infrared spectroscopy. The crystallization process occurs in two stages; firstly, the particles of ACC rapidly dehydrate and crystallize to form individual particles of vaterite; secondly, the vaterite transforms to calcitevia a dissolution and reprecipitation mechanism with the reaction rate controlled by the surface area of calcite. The second stage of the reaction is approximately 10 times slower than the first. Activation energies of calcite nucleation and crystallization are 73 ± 10 and 66 ± 2 kJ mol−1, respectively. A model to calculate the degree of calcite crystallization from ACC at environmentally relevant temperatures (7.5–40 °C) is also presented.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...