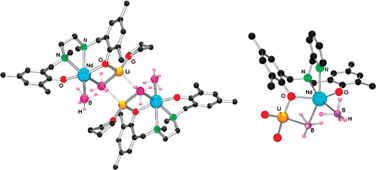
New heterobimetallic borohydrido neodymium complexes {[OONN]1Nd(BH4)(μ-BH4)Li(THF)}2 (1) and [OONN]3Nd(BH4)(μ-BH4)Li(THF)2 (3) supported by diamino-bis(phenoxide) ligands ([OONN]1 = {CH2N(Me)CH2-3,5-Me,t-Bu-C6H2O}2; [OONN]3 = C5H4NCH2N{CH2-3,5-Me,t-Bu-C6H2O}2) were synthesized by the reactions of Nd(BH4)3(THF)2 with equimolar amounts of dilithium derivatives of diamino-bis(phenol)s Li2[OONN]n and isolated in high yields. In the case of Li2[OONN]2 ([OONN]2 = Me2NCH2CH2N{CH2-3,5-t-Bu-C6H2O}2), the same synthetic procedure afforded the heterobimetallic bis(phenoxide) complex Li{Nd[OONN2]2} (2). The structures of complexes 1–3 were established by X-ray diffraction studies. Compounds 1–3 act as single-site initiators for the ring-opening polymerization (ROP) of racemic lactide and racemic β-butyrolactone under mild conditions (20 °C), providing atactic polymers with controlled molecular weights and relatively narrow polydispersities (Mw/Mn = 1.07–1.82). While 1 and 3 initiate polymerizationvia their borohydride groups, ROP with 2 proceeds viainsertion into the Nd–O(ligand) bond.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...