Role of metal ions in aggregation of intrinsically disordered proteins in neurodegenerative diseases†
Abstract
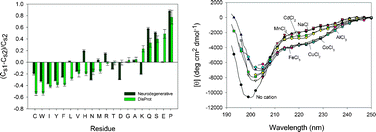
Neurodegenerative diseases constitute a set of pathological conditions originating from the slow, irreversible, and systematic cell loss within the various regions of the brain and/or the spinal cord. Depending on the affected region, the outcomes of the neurodegeneration are very broad and diverse, ranging from the problems with movements to dementia. Some neurodegenerative diseases are associated with
- This article is part of the themed collection: Metal Toxicity

 Please wait while we load your content...
Please wait while we load your content...