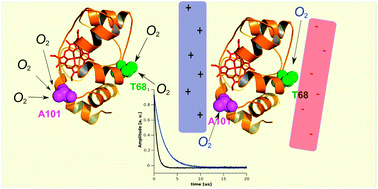
Reorientation of cytochrome c2 upon interaction with oppositely charged macromolecules probed by SR EPR: implications for the role of dipole moment to facilitate collisions in proper configuration for electron transfer†
Abstract
The reaction of water-soluble
- This article is part of the themed collection: Cytochromes

 Please wait while we load your content...
Please wait while we load your content...