Abstract
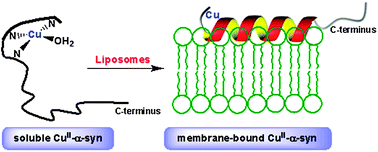
Interactions of copper and membranes with α-synuclein have been implicated in pathogenic mechanisms of Parkinson's disease, yet work examining both concurrently is scarce. We have examined the effect of copper(II) on
- This article is part of the themed collection: Metals in Neurodegenerative Disease

 Please wait while we load your content...
Please wait while we load your content...