Design, synthesis and antiproliferative activity of urocanic-chalcone hybrid derivatives†
Abstract
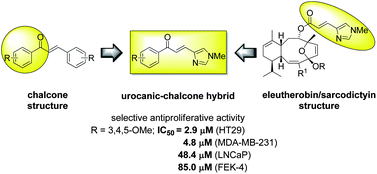
Inspired by biologically active natural products, a hybrid analogue that combines the N-Me urocanic side chain of the sarcodictyin family of compounds with the

 Please wait while we load your content...
Please wait while we load your content...