Metal preference of Zn(ii) and Co(ii) for the dinuclear metal binding site of IMP-1 metallo-β-lactamase and spectroscopic properties of Co(ii)-substituted IMP-1 with mercaptoacetic acid†
Abstract
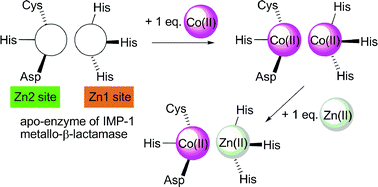
IMP-1 metallo-β-lactamase is a dinuclear Zn(II) enzyme that catalyzes the hydrolysis and inactivation of most β-lactams including
 Please wait while we load your content...
Please wait while we load your content...