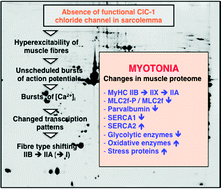
Myotonia is a symptom of various genetic and acquired skeletal muscular disorders and is characterized by hyperexcitability of the sarcolemma. Here, we have performed a comparative proteomic study of the genetic mouse models ADR, MTO and MTO*5J of human congenital myotonia in order to determine myotonia-specific changes in the global protein complement of gastrocnemius muscle. Proteomic analyses of myotonia in the mouse, which is caused by mutations in the gene encoding the muscular chloride channel Clc1, revealed a generally perturbed protein expression pattern in severely affected ADR and MTO muscle, but less pronounced alterations in mildly diseased MTO*5J mice. Alterations were found in major metabolic pathways, the contractile machinery, ion handling elements, the cellular stress response and cell signaling mechanisms, clearly confirming a glycolytic-to-oxidative transformation process in myotonic fast muscle. In the long-term, a detailed biomarker signature of myotonia will improve our understanding of the pathobiochemical processes underlying this disorder and be helpful in determining how a single mutation in a tissue-specific gene can trigger severe downstream effects on the expression levels of a very large number of genes in contractile tissues.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...