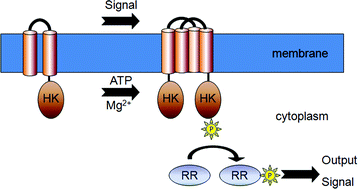
Two-component signal transduction systems are found ubiquitously in prokaryotes, and in archaea, fungi, yeast and some plants, where they regulate physiologic and molecular processes at both transcriptional and post-transcriptional levels. Two-component systems sense changes in environmental conditions when a specific ligand binds to the receptor domain of the histidine kinase sensory component. The structures of many histidine kinase receptors are known, including those which sense extracellular and cytoplasmic signals. In this review, we discuss the basic architecture of two-component signalling circuits, including known system ligands, structure and function of both receptor and signalling domains, the chemistry of phosphotransfer, and cross-talk between different two-component pathways. Given the importance of these systems in regulating cellular responses, many biochemical techniques have been developed for their study and analysis. We therefore also review current methods used to study two-component signalling, including a new affinity-based proteomics approach used to study inducible resistance to the antibiotic vancomycin through the VanSR two-component signal transduction system.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...