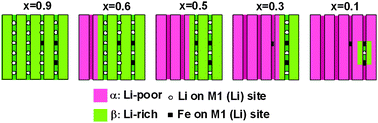
Solid solutions of LixFePO4 are of tremendous interest because of a proposed increase in ion transport properties, but the formation of these solutions at high temperatures is difficult if not impossible and direct synthesis is difficult and rarely reported. Here we report modified polyol syntheses which produce nanocrystalline Li1−yFePO4 directly, where the maximum Li substoichiometry on the M1 site sustained at synthesis temperatures of 320 °C is about 10%. High target lithium vacancy concentrations promote the increase in anti-site disorder of Li+ and Fe2+, as this process is driven by vacancy stabilization. Combined neutron and X-ray diffraction on partial delithiated substoichiometric olivines reveals segregated defect-free (where Li is extracted) and defect-ridden (where Li remains) regions. This proves (1) that the anti-site defects obstruct Li+ diffusion explaining the detrimental electrochemistry and (2) that the anti-site defects form clusters. Finally, preferential anisotropic strain broadening in the bc-plane indicates the existence of a coherent interface between the Li-poor and Li-rich phases. Along with the size broadening upon delithiation this proves that in nano-sized LixFePO4 the two phases coexist within a single particle, which is not expected based on thermodynamics arguments due to the energy penalty associated with the coherent interface. Thereby, these results give important and unique insight and understanding in the properties of nano sized LiFePO4.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...