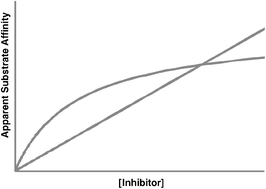
Limitations of conventional inhibitor classifications
Abstract
* Corresponding authors
a
Department of Chemistry, Carleton University, Ottawa, Ontario, Canada
E-mail:
rwalsh@connect.carleton.ca
b Department of Chemistry, Mount Saint Vincent University, Halifax, Nova Scotia, Canada
c Department of Anatomy & Neurobiology Medicine (Neurology and Geriatric Medicine), Dalhousie University, Halifax, Nova Scotia, Canada

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
