pH-Responsive chelating N-heterocyclic dicarbene palladium(ii) complexes: recoverable precatalysts for Suzuki–Miyaura reaction in pure water†
Abstract
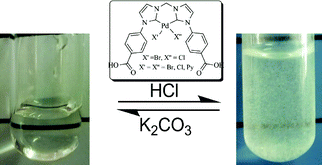
A hydrophobic palladium complex of the chelating N-heterocyclic dicarbene (NHDC) (3) was synthesized through in situ deprotonation of a bisimidazolium salt (2) by Pd(OAc)2 in

 Please wait while we load your content...
Please wait while we load your content...