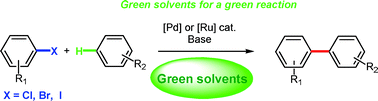
The formation of carbon-carbon bonds by transition metal-catalysed direct functionalisation of C–H bonds has recently emerged as a greener alternative to traditional cross-coupling reactions. Despite tremendous improvement of the catalytic efficiency allowing reactions under milder conditions or lower catalyst loadings, this type of transformation still suffers from a major limitation with regards to green chemistry concerning the reaction media since the preferred solvents for these transformations are the undesirable and toxic, high boiling point, polar, aprotic, N-methylpyrrolidinone (NMP), dimethylformamide (DMF) or dimethylacetamide (DMAc). Recently, efforts have been made to perform these reactions in greener or more environmentally acceptable media. This review summarises the contributions made in this direction during the past few years.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...