New stable aryl-substituted acyclic imino-N-heterocyclic carbene: synthesis, characterisation and coordination to early transition metals†
Abstract
The synthesis of the bulky 1-(1-arylimino-2,2-dimethylpropyl)-3-(aryl)imidazolium salt from the corresponding
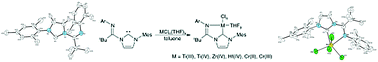

 Please wait while we load your content...
Please wait while we load your content...